Natural products are a unique collection of small molecules that offer invaluable starting points for uncovering, understanding and modulating unknown biological processes. They also serve as inspiration for developing new synthetic strategies and methodologies. Our lab invents transformative chemistry to enable concise, scalable and modular natural product synthesis, unlocking their medicinal potential. We are also committed to training the next generation of scientific warriors, prepared to tackle challenges at the dynamic interface of chemistry and biology.
Harnessing polyfunctional intermediates with orthogonal reactive groups to achieve chemoselective natural product synthesis
Steroids, triterpenes and limonoids are a class of polycyclic natural products that continuously offer many opportunities for drug discovery. Our pursuit leverages a strategically designed, polyfunctional intermediate featuring orthogonal reactive groups, from which direct and modular C–C bond formations to access complex polycyclic triterpene scaffolds. Such a planning minimizes protecting group and redox manipulations, and enables concise syntheses of a series of triterpenes and limonoids. Glycinoeclepin A, for exmaple, a scarce yet extremely potent soybean cyst nematode hatching stimulus that previous require 25-39 steps, was prepared in merely 15 steps.
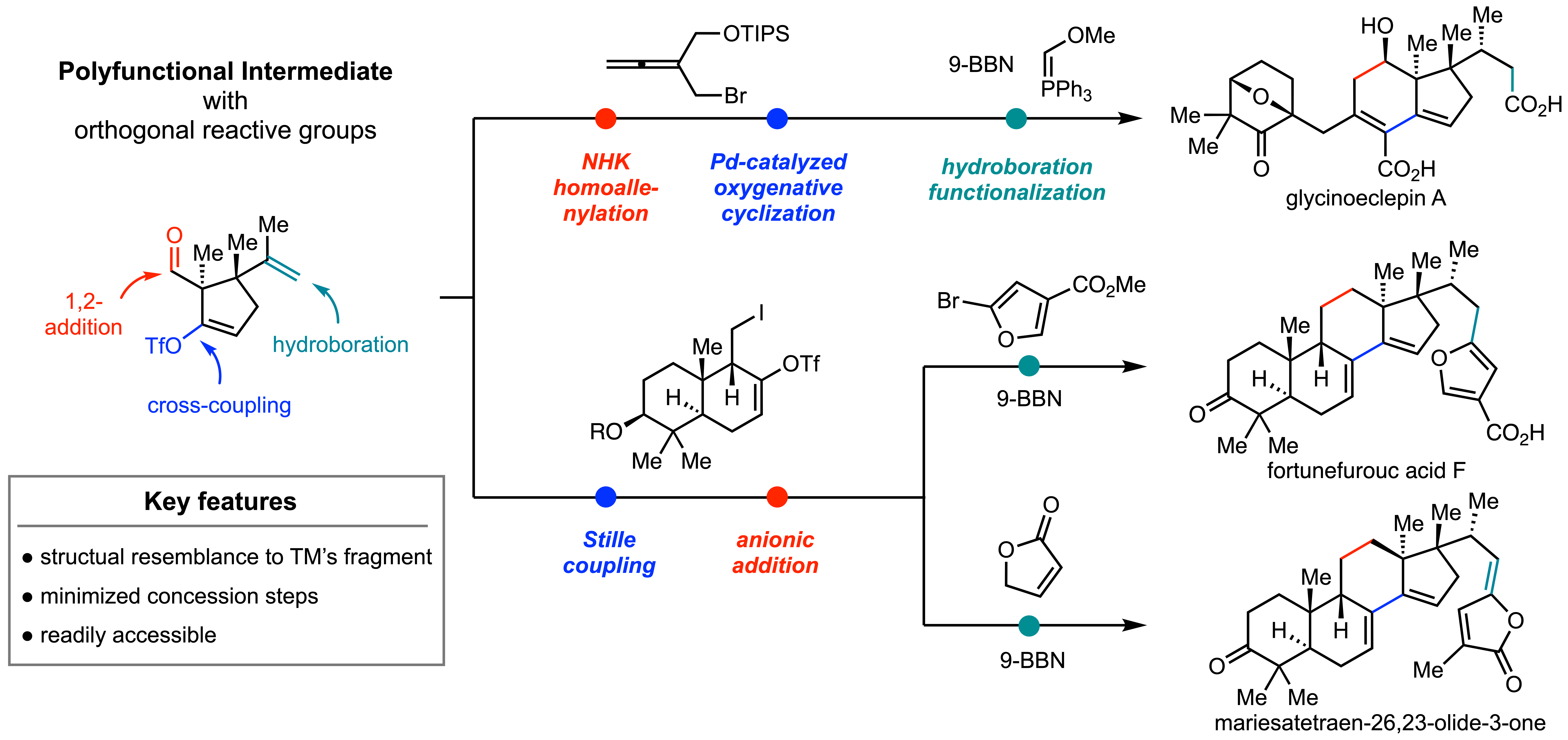
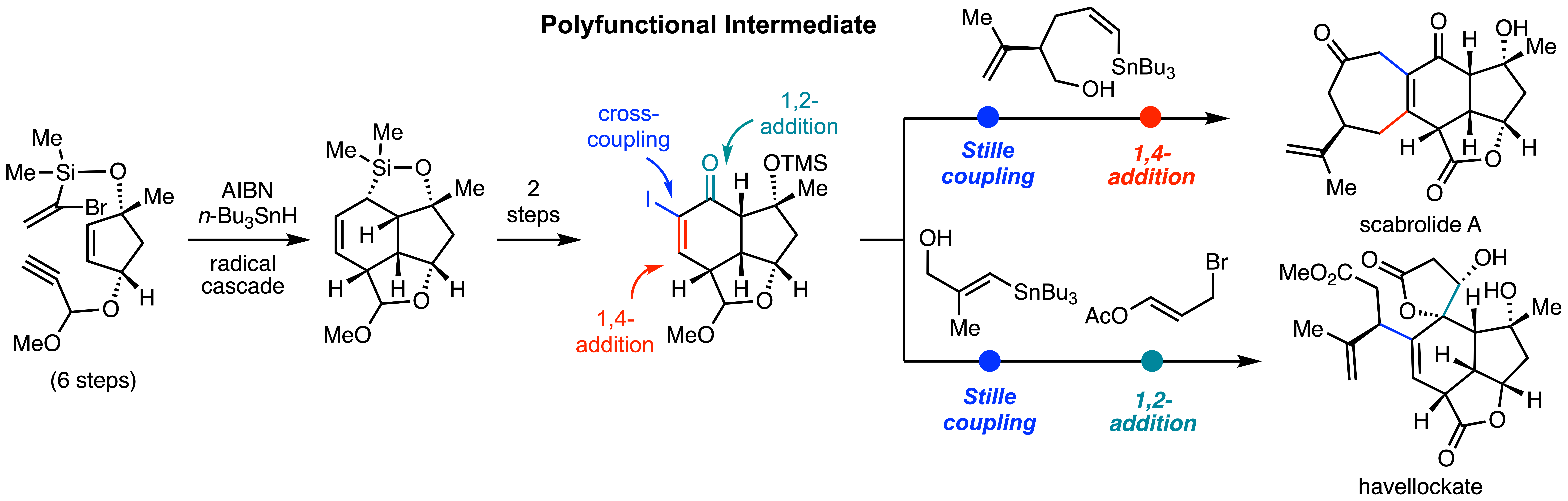
This strategy is broadly applicable to other types of naturally occuring molecules, including norditerpenoid scabrolide A and havellockate.
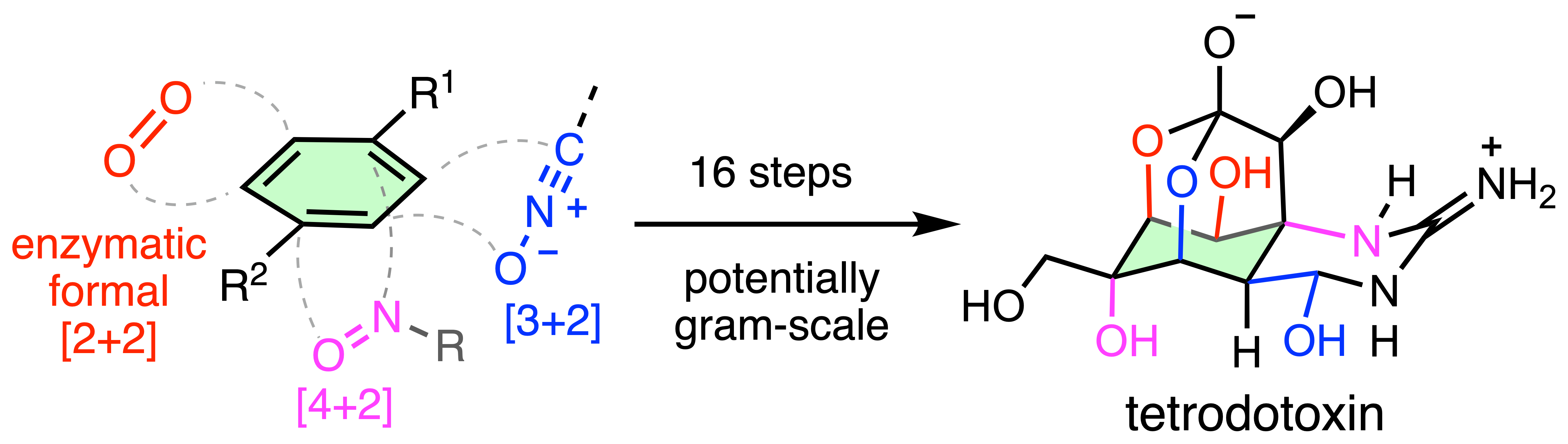
Chemoenzymatic approach to synthesize densely functionalized natural products
We are also actively pursuing natural products that incorporate multiple heteroatom substituents in a compact framework (i.e. tetrodotoxin). The skeletal rigidity and dense functionalization can result in significant bioactivities, but also immense synthetic challenges. We developed a chemoenzymatic approach to tackle these diffculties. Through strategically merging arene dioxygenation catalyzed by engineered toluene dioxygenase (TDO), together with two chemical cycloaddition, fragmentation sequences, we were able to rapidly construct the octa-substituted cyclohexane core of tetrodotoxin and completed this celebrated alkaloid in 16 steps on gram scale. This overall strategy can be readily applicable to other complex molecules with a series of projects ongoing in our lab.
(For details of these works, see our publications)


